Estimated reading time: 9 minutes
The risk of climate change is rising all over the world, and now it’s affecting our drinking water. You’d think that with a planet that is roughly 70 percent covered in water that most populations would have access to potable sources.
The main problem is that the majority of water available is saltwater. It is widely believed that only about 3 percent of the available water is freshwater. What’s more, is that roughly two-thirds of that freshwater is locked away in glaciers where it can't be accessed. It is because of this paradox that over a billion people don’t have access to fresh water, many of which live by the sea.
Increased regional water scarcity has forced us to find ways to utilize salty ocean water for hydration, but it is still not widely used. If converting saltwater into freshwater is an option, why aren’t we doing more of it?
Let’s look at the different methods to desalinate water and talk a little bit about the obstacles that need to be overcome for it to be a sustainable practice of getting fresh water.
Want to save this post for later? Click Here to Pin It On Pinterest!
Why We Can’t Drink Salt Water
You’d think that because salt is part of our diet that we would be able to process salt water, right? Ultimately, that isn’t the truth as the salt content in seawater is much too high for our kidneys to process regularly.
Human kidneys produce urine that is salty by nature, although much less salty than the ocean. Drinking fresh water helps balance the amount of salt in your body by letting the kidneys flush any excess salt out.
Drinking saltwater inhibits their ability to function because you are simply adding too much salt to your system. The more salt water you drink, the more salt in your body, and the less your kidneys can function.
You can quickly see how this will lead to dehydration. This effect is further compounded and exacerbated if the person has health issues such as kidney disease or is on a restricted salt diet.
This is an all too common problem in Uruguay as the region is warming up at an unprecedented rate, causing their fresh water supplies to only be at 5 percent capacity.
A decision was made by the State Sanitary Works Administration to start adding saltwater to their supply to help bolster their reserves. As you can guess, the results didn’t do much: sodium levels have skyrocketed to 10 times their historic levels. It is leading to widespread health problems such as high blood pressure, especially among the immunocompromised.
In their case, a desalination plant would be the only effective means to get fresh water to the population. The primary issue is that the energy costs to maintain that project would be large, and why it more than likely wasn’t thought of initially.
What is Desalination?

When desalination is applied to seawater it eliminates all of the dissolved solids, including salt. Currently, it is the most used process to get fresh water for agriculture and human consumption.
Humans didn’t create this process, we just copied it from nature. When seawater evaporates it creates rain clouds and the salt is left behind. That condensed water from the rain clouds is fresh. Famous figures such as Aristotle and Leonardo Da Vinci learned this back in their respective times.
There are two main types of desalination, including thermal distillation and separation through a membrane. Thermal distillation uses heat to turn water into a vapor that is collected and condensed into water. This process leaves the salt behind as the water evaporates.
The second type, membrane separation is commonly seen in reverse osmosis systems. In this method, the water is forced through a semipermeable membrane that separates the salt from the water.
Historically, desalination is a process commonly seen on boats or submarines. Water supplies need to be replenished and it’s the only way to do it at sea. Now entire factories that can create fresh water from the sea have become the natural evolution. You can even find reverse osmosis systems in many people's homes as well as commercial agriculture operations.
5 Ways to Turn Saltwater into Drinkable Water
More and more methods of desalination are being invented and it can get pretty technical. These 6 methods combine primitive and advanced ways for you to utilize the salty seawater to create your potable resource.
Solar Distillation

Closest to the natural way, solar distillation mimics the evaporation cycle on a much smaller scale. Using this method is scalable and there are even factories that evaporate large amounts of seawater currently.
The easiest way to build a solar still is to wait for a sunny day, dig a hole in the ground, and line it with plastic. Pour in the saltwater to be desalinated and place a cup or container in the middle of the hole. Cover the hole with a sheet of plastic and place a heavy rock right in the middle, directly above the cup you left in it.
As the sun heats the hole you just dug the saltwater will start to evaporate. The vapor gets caught on the plastic roof and condenses into water, leaving the salt behind.
The rock on the top of the plastic helps direct the condensed water into the cup or container you left in the hole. After a bit of time, you should be able to check the hole to find some water in the catch cup.
This is the simplest and most primitive way to desalinate water. The amount of fresh water you get is dependent on the size of the hole and the amount of seawater you are filling it with.
Pot and Stove Method
This method can be considered forced distillation as you don’t rely on the sun, but your stove at home. All you need is a large pot (preferably with a glass lid), your stove, and a thick drinking glass.
The idea is similar to solar still in that you fill a pot with seawater and place the drinking glass in the middle. Don’t overflow it as the saltwater might contaminate the fresh water that will be in the glass.
Crank up the heat and place the pot lid inverted, meaning the handle is facing down. It should be directly above the cup that you had placed in the pot before boiling.
As the vapor rises from the seawater it will catch and condense on the pot lid. Since most pot lids are dome-shaped it will start to run down the lid and the handle, dripping into the drinking glass. Wait for the water to cool down and it’ll be salt-free.
Reverse Osmosis
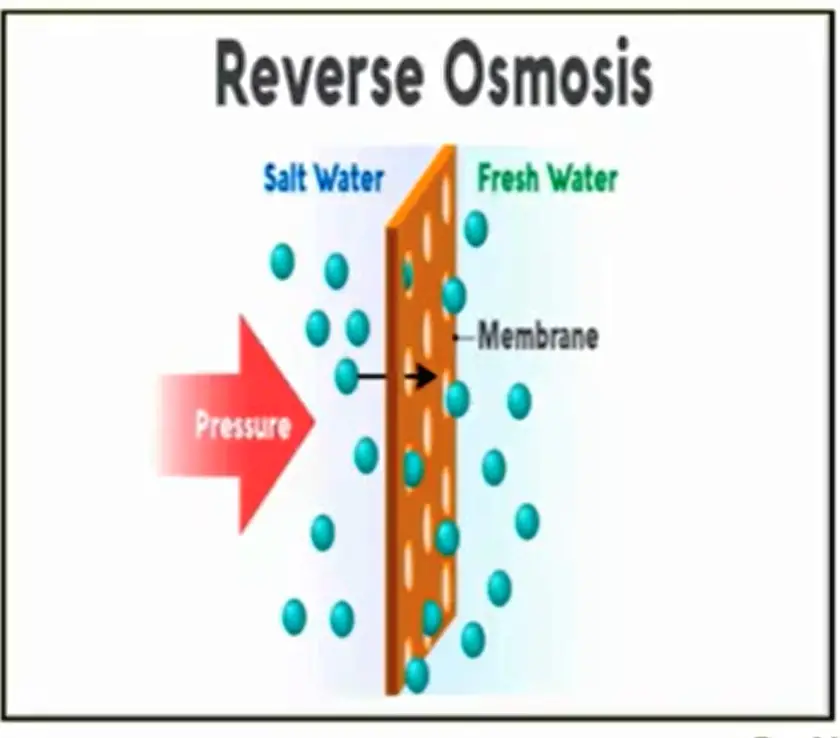
Reverse osmosis is the most advanced and commonly used desalination process both commercially and for residential needs. The process uses a semi-permeable material which is used to filter the salt out of the seawater.
The science behind it is interesting as the membrane allows the pure water to pass through while stopping the salt ions.
The fresh water is then remineralized and purified and sent to a tank for storage. The salt left behind is diluted and then taken out of the system. Large facilities will dilute the remaining salt and push it right back out into the ocean.
Nanofiltration

Nanofiltration hasn’t quite taken off yet but is still an incredibly viable way to get purified water. Much like reverse osmosis, nano filter membranes are semi-permeable. Unlike reverse osmosis, they are more permeable which allows more water to be processed at once.
The main draw is the amount of water that they can process in such a small space. This makes it potentially cost-effective and efficient for commercial use.
In addition to salt, the membranes are manufactured using sulfonated components such as graphene oxide that will remove all traces of pollutants, both chemical and naturally occurring.
Electrodialysis Reversal Desalination
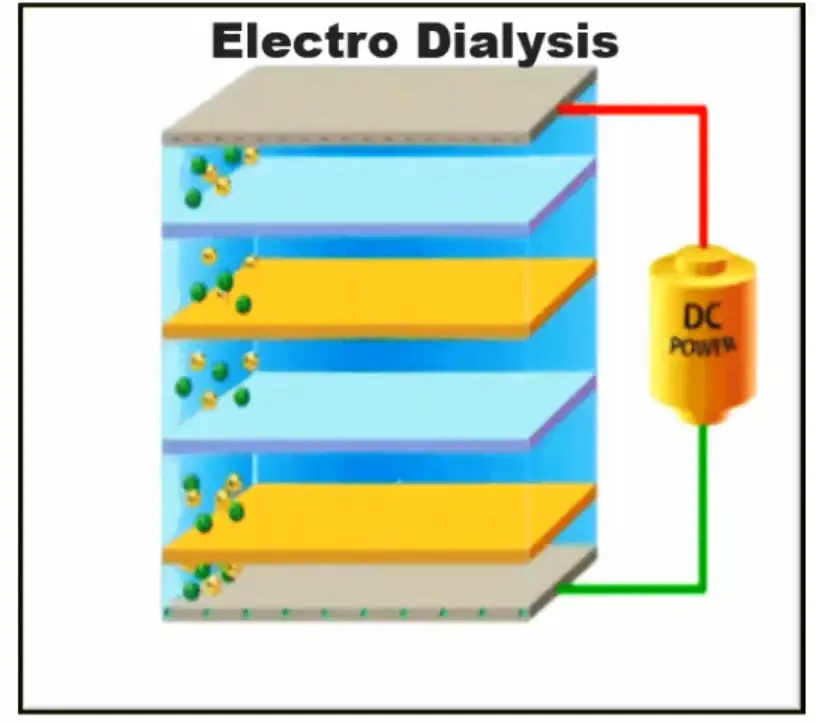
Probably the most complicated method on the list and only used by commercial projects, EDR uses the power of electricity to purify seawater for consumption.
The process works by applying electricity to an ion exchange membrane (much like reverse osmosis and nanofiltration) to pull the salts from the water. The water is then separated into a high and low sodium content stream with the low going to storage.
While you probably won’t see this process available to the public anytime soon, the technology is advancing quickly and desalination through electrolysis might be a cost-effect solution to a water crisis.
Should You Purify the Water Before Drinking?
Condensed water is considered pure water as nothing else can evaporate with it. If your container is contaminated, however, it can lead to ruining your fresh water source.
Any of the methods using distillation should be safe for you to drink without further purification. This is mainly due to minerals, bacteria, and viruses being unable to evaporate with the water.
To ease your nerves you can run it through another filter such as a Lifestraw, but generally condensed water should be safe for consumption.
Like this post? Don't Forget to Pin It On Pinterest!









